you are analyzing a compound in the laboratory|Biology chapter 6.4 Flashcards : private label You are analyzing a compound in the laboratory. You find that it is made up of carbon, hydrogen, and oxygen in a ratio of 1:2:1. How will you classify the compound? web21 de abr. de 2022 · Click here to Subscribe! http://bit.ly/SUBJESTERSubscribe to my SECOND channel http://bit.ly/SUBJESSE_BARONInstagram: @jesse_baron» .
{plog:ftitle_list}
Emily Vick photos & videos. EroMe is the best place to share .
You are analyzing a compound in a laboratory. You find that it is made up of carbon, hydrogen, and oxygen in a ratio of two hydrogen atoms for each carbon atom. How will you classify the .You are analyzing a compound in the laboratory. You find that it is made up of carbon, hydrogen, and oxygen in a ratio of two hydrogen atoms for each carbon atom. How will you classify the .
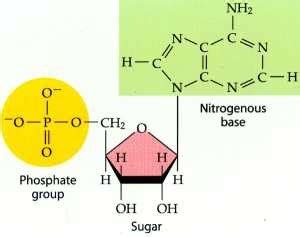
You are analyzing a compound in the laboratory. You find that it is made up of carbon, hydrogen, and oxygen in a ratio of 1:2:1. How will you classify the compound?The compound composed of carbon, hydrogen, and oxygen in a ratio of 2:1 for hydrogen to carbon and oxygen atoms is likely a monosaccharide. These are simple sugars that serve as the basic building blocks of carbohydrates. Their general formula is (CH 2 _2 2 O) n _n n , where "n" denotes the number of carbon atoms present. 1. The compound described in the question, which contains carbon, hydrogen, and oxygen in a ratio of two hydrogen atoms for each carbon atom, can be classified as a carbohydrate. Carbohydrates are organic compounds made up of carbon, hydrogen, and oxygen atoms in specific ratios.
Synthesis and Analysis of Cooridnation Compounds 1 Experiment 13 Synthesis and Analysis of Coordination Compounds Pre-Lab Assignment Before coming to lab: • Read the lab thoroughly. • Answer the pre-lab questions that appear at the end of this lab exercise. The questions should be answered on a separate (new) page of your lab notebook. You are analyzing a compound in the laboratory. You find that it is made up of carbon, hydrogen, and oxygen in. Get the answers you need, now! Anandkumar54271 Anandkumar54271 02.11.2021 Biology Secondary School answered • expert verified You are analyzing a compound in the laboratory.
You are analyzing a compound in a laboratory. you find that it is made up of carbon, hydrogen, and oxygen in a ratio of two hydrogen atoms for each carbon atom. how will you classify the compound?
In this lab we will focus on using Solubility Tests, Chemical Tests and Spectra Analysis to identify two unknown compounds. Overview: In this experiment, you will combine both spectroscopy and qualitative tests to identify an . In Part B of the lab (Week 2), you will conduct a series of experiments to distinguish between the The usual recipe is a bit more complex here, but nothing that you won’t find around in any lab. You basically need to dissolve 1.5 g of potassium permanganate and 10 g of potassium carbonate in 200 mL of water. To this mixture, add in 1 mL of 10% aqueous NaOH, and stir. . Analyze the results: Any compound that is stable in silica gel, will .Most compounds that we handle in the o-chem labs at PSU should give a single spot on the TLC plate. Ideally, each compound in a mixture will produce a distinct spot so a sample with two compounds will give two different spots, and so on. An important property of any compound, is its R f-value (retention factor). In simple terms, this value is .
Study with Quizlet and memorize flashcards containing terms like You are analyzing a compound in the laboratory. You find that it is made up of carbon, hydrogen, and oxygen in a ratio of Two Hydrogen Atoms for each Carbon atom. How will you classify the compound?, Water is essential for life. Its special properties make water the single most important molecule .
It's a carbohydrate! Well funnily the way the question is phrased: you are a carbohydrate, since you are the compound The question states the compound is made up of only carbon, oxygen and hydrogen, therefore it narrows it down to Lipids or carbohydrates as a macromolecule choice. Carbohydrates have a ratio of 2 hydrogen atoms for each oxygen and .Question: You are working in a science lab that is analyzing the contents of cow manure. You discover a novel compund not seen in other cow manure samples made entirely of C, H, and O. When 33.03 g of this compund are burned, .You are analyzing a compound in the laboratory. You find that it is made up of carbon, hydrogen, and oxygen in a ratio of two hydrogen atoms for each carbon atom and gives you a quick energy source. How will you classify the compound? lipid. protein. carbohydrate. nucleic acid. 3. Multiple Choice. You are analyzing a compound in a laboratory. You find that it is made up of carbon, hydrogen, and oxygen in a ratio of two hydrogen atoms for each carbon atom. How will you classify the compound? Question 19 options: carbohydrate lipid protein nucleic acid
a.You run a column on a mixture of two unknown halogenated alkenes, but hey both elute from the column at the same time. The solvent used was methylene choride (dichloromethane). b.You run an alumina column on a mixture of a thiol and an amine, but you never get any compound off the column (i.e. neither compound elutes form the column).
you suspect that a chemical that you are testing in the lab is strongly acidic. what might be is pH. 2. the isotopes carbon-12, carbon-13 and carbon-14 have different number of. . You are analyzing a compound in a laboratory. You .1) a way to tell if compound is pure/to determine the effectiveness of a purification 2) a way to see what compounds are in a sample by comparison 3) monitor progress of a reaction (By sampling a reaction from time to time, you .You are analyzing a compound in a laboratory. You find that it is made up of carbon, hydrogen, and oxygen in a ratio of two hydrogen atoms for each carbon atom. How will you classify the compound? carbohydrate. See an expert-written answer! We have an expert-written solution to this problem!
Pre-Laboratory assignment for "Synthesis and Analysis of a Coordination Compound" Part 1 1.A nickel coordination compound, NI(NH3)o2, can be prepared by a procedure similar to the one you will use to prepare Cu(NH34so4 H20, The reaction can be represented stoichiometrically as NiCI2 6H20+6 NH4OH NiNH3)62+ 12 H20. 4.850 g of NiCl2: 6H20 is treated with 22.0 mL of .
Study with Quizlet and memorize flashcards containing terms like You are analyzing a compound in the laboratory. You find that it is made up of carbon, hydrogen, and oxygen in a ratio of two hydrogen atoms for each carbon atom. How will you classify the compound?, Lipids can be digested into what smaller subunits?, Many plants have waxy coatings on some surfaces.
compound. Operational goal: Develop the skills to take the melting point of a compound effectively, including proper packing of a sample in a melting point capillary tube, precise determination of the melting point range of a sample. Introduction In this laboratory you will identify an unknown compound by using mixture melting points.You are analyzing a compound in the laboratory. You find that it is made up of carbon, hydrogen, and oxygen in a ratio of two hydrogen atoms for each carbon atom. How will you classify the compound? carbohydrate Carbohydrates are made up of Carbon, hydrogen, and oxygen. See the video for the basic carbohydrate structure.Your lab is analyzing the materials found on a planet close to the main red dwarf star. You find a compound made entirely of C, H, and O. When 23.21 g of this compound are burned, you recover 35.21 g of CO2, and 7.21 g of H2 What is the empirical formula of this compound?
UNKNOWN REPORTS. You should submit an Unknown Preliminary Report as soon as you have completed the experiments described below:. For the solid unknown, you will take an accurate mp, an IR spectrum using the KBr procedure, solubility tests and, based upon an analysis of this information, do specific functionality tests as described in the manual.If you suspect that you .You are analyzing a compound in the laboratory. You find that it is made up of carbon, hydrogen, and oxygen in a ratio of two hydrogen atoms for each carbon atom. How will you classify the compound? lipid. protein. carbohydrate. nucleic acid. 3. Multiple Choice. Edit. 1 minute. 1 pt. Fats, oils and cholesterol are all types of what? cell .(TLC) is a method for analyzing mixtures by separating the compounds in the mixture based on polarity. TLC can be used to help determine the number of components in a . Prelab: You may either print out your prelab and bring it with you to lab, or bring your computer. Your TA will grade it on the spot before you begin the experiment.
Chemical scientists want to explore the natural world and identify all its chemical components. They also want and need to identify all of the new chemical substances produced directly and indirectly as a result of their synthetic and manufacturing endeavors. As described in Chapter 7, much of this has involved learning the nature of the substances that are part of, and produced .You will learn how NMR spectroscopy works and how chemists use this method to analyze compounds in organic chemistry! Topics Covered in Other Articles. Resonance (a separate . or protons. It is the most commonly used in the laboratory. 1 H NMR can give you information on several topics: Amount of unique hydrogens in the compound; Ratios of .
Biology chapter 6.4 Flashcards

riehle portable hardness tester wilson rockwell
riehle portable rockwell hardness tester
みんながダウンロード保存した人気のTwitter動画 | TWIVIDE.
you are analyzing a compound in the laboratory|Biology chapter 6.4 Flashcards